Procedure for Preparation of Culture Media
1. Preparation of Culture Media
i. 37 g of the medium is suspended in 1 L distilled water and boiled to dissolve.
ii. Autoclave at 121 °C, 15 psi for 15 min.
13 g dissolved in 1 L distilled water. Autoclave at 121 °C for 15 min.
i. 55 g in 1 L water; boil to dissolve.
ii. Autoclave 121 °C, 15 psi for 15 min.
i. 42.5 g base in 1 L water; autoclave at 121 °C for 15 min.
ii. Cool to 40–50 °C, add 50 mL sterile sheep blood, mix, pour plates.
i. Pour sterilized BA into plates.
ii. Incubate 75 °C for 30 min; color turns chocolate brown.
i. 38 g in 1 L water; warm to dissolve.
ii. Dispense 10 mL in tubes; boil in water bath 10 min.
2. Preparation of Biochemical Media
i. 9.4 g in 1 L cold water; boil to dissolve.
ii. Autoclave 121 °C, 15 psi for 15 min.
iii. Aseptically add 10 mL sterile dextrose per 100 mL medium; dispense into tubes.
i. 36 g in 1 L water; dissolve fully.
ii. Dispense to 3 inches depth in tubes; sterilize by autoclave.
i. 17 g in 1 L water.
ii. Dispense 3 mL per tube; autoclave 121 °C for 15 min.
i. 24.2 g in 1 L water.
ii. Dispense 3 mL per tube; autoclave 121 °C for 15 min; tilt tubes to form slants.
i. 65 g in 1 L water; autoclave 121 °C, 15 psi for 15 min.
ii. Pour slants with 1 in butt depth.
i. 24 g in 950 mL water; autoclave 121 °C for 15 min.
ii. Cool to ~45 °C; add 50 mL 40% urea; dispense as slants.
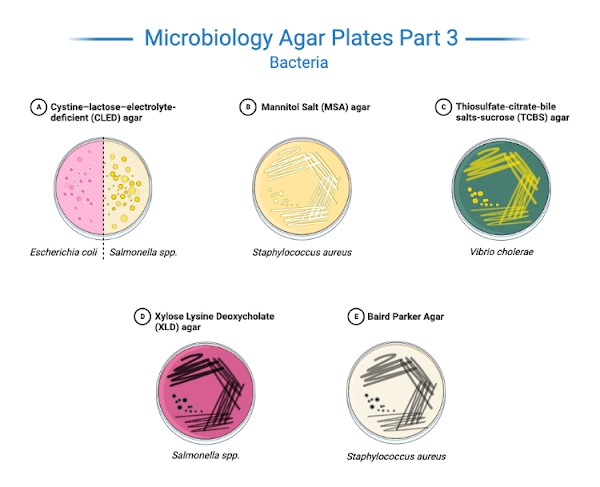
Some Common Agar Plates


Template Source: Created with Biorender.com
No comments:
Post a Comment